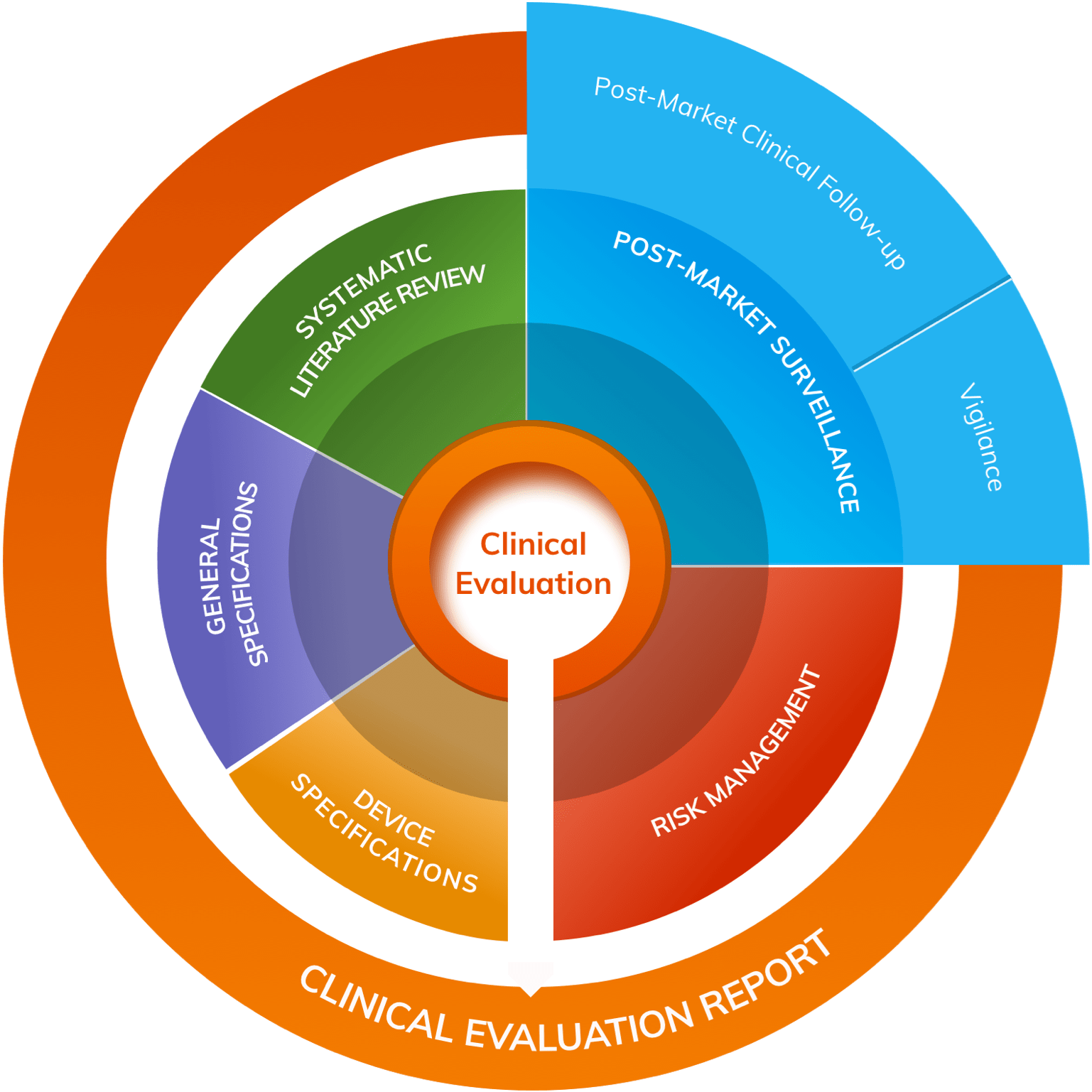
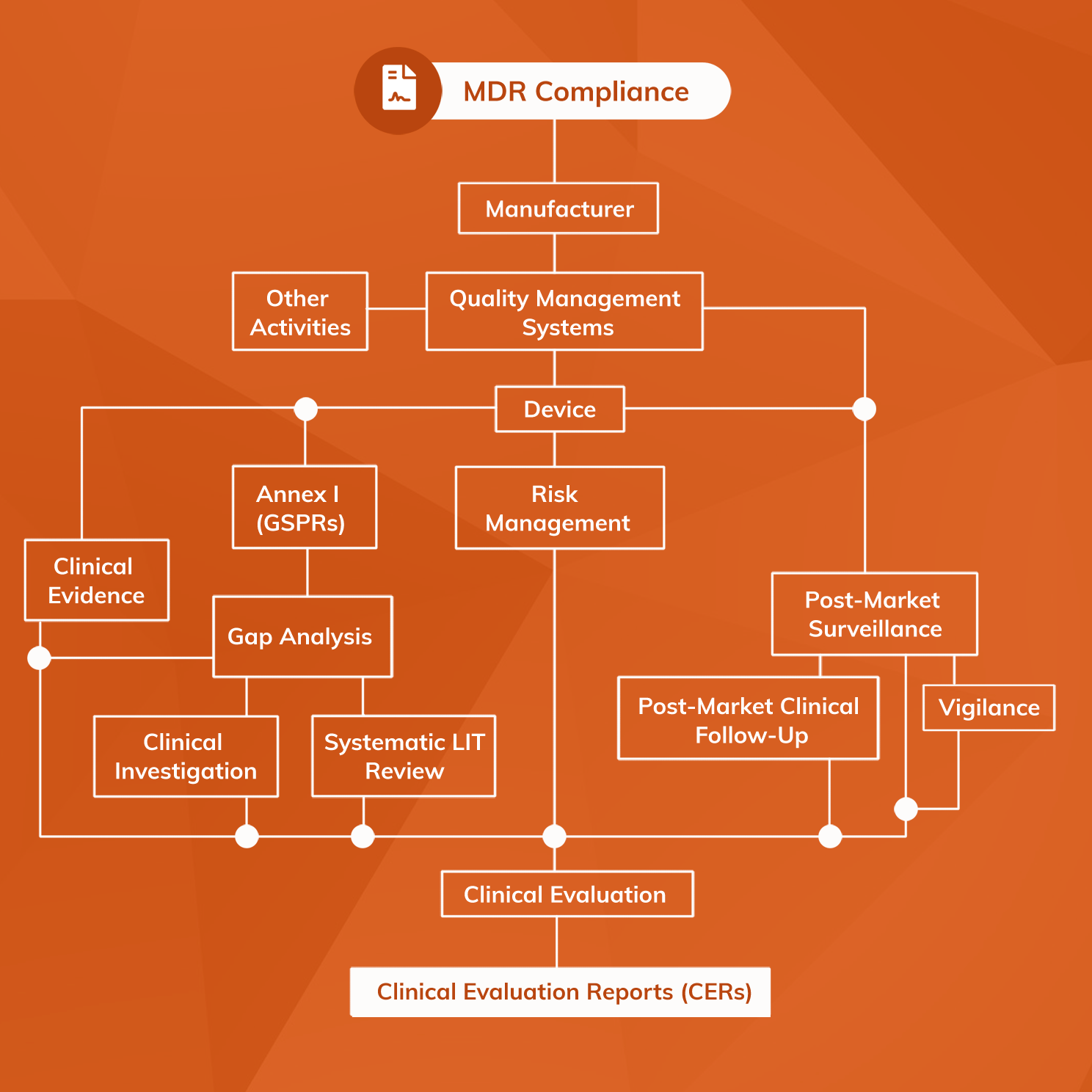
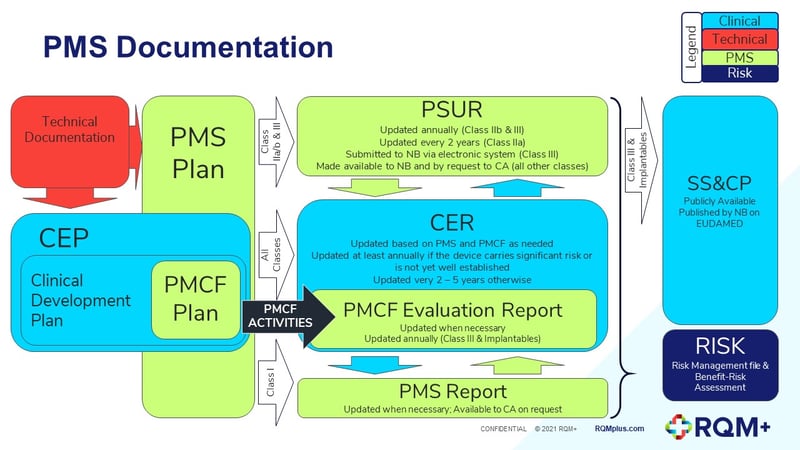
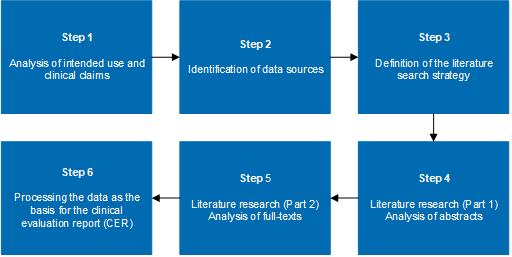
Generis Group on Twitter: "Looking to find out about some of the most critical and new #MDR documents, and how they're interconnected — all in one place? Learn more with @CactusLifeSci: https://t.co/vIRc9s1ZjZ -- #

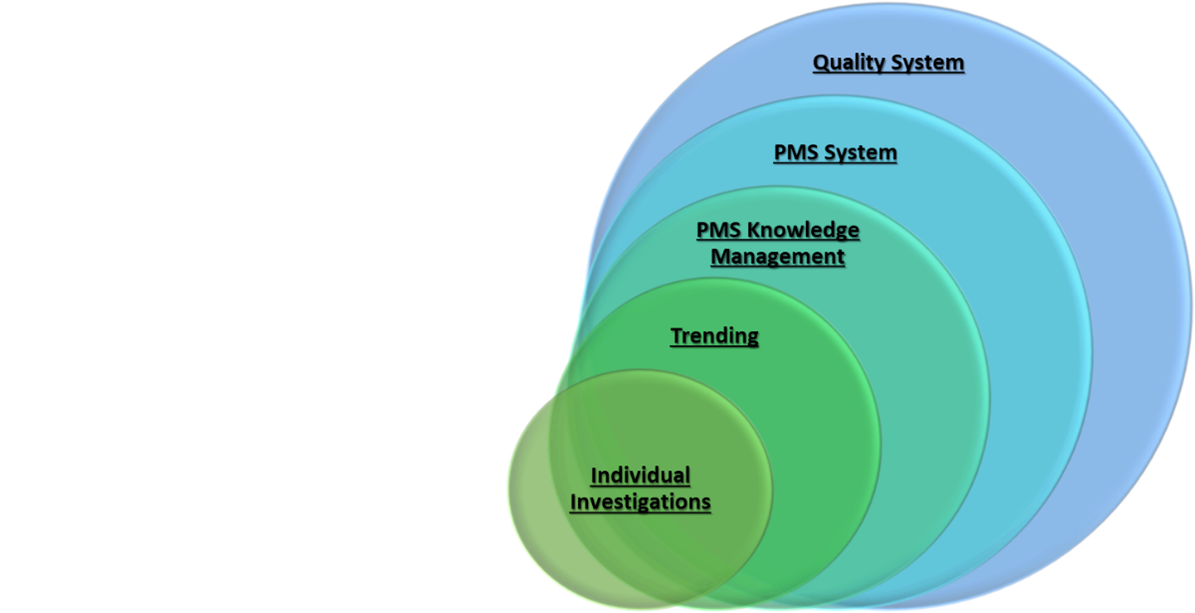
Novelties for Clinical Evaluation and Post Market Surveillance within MDR - the Posts Series - confinis