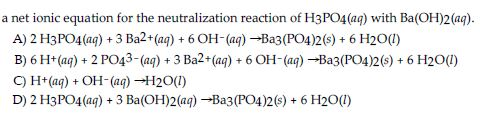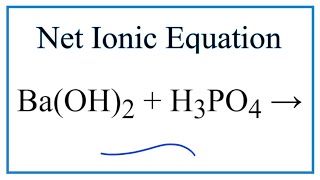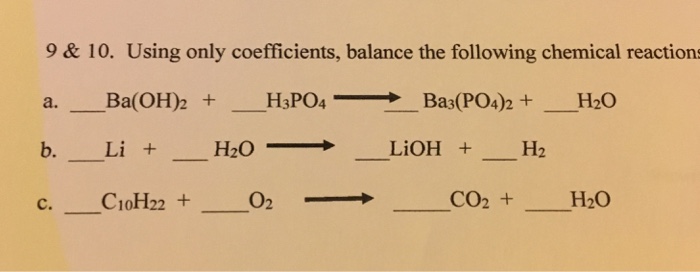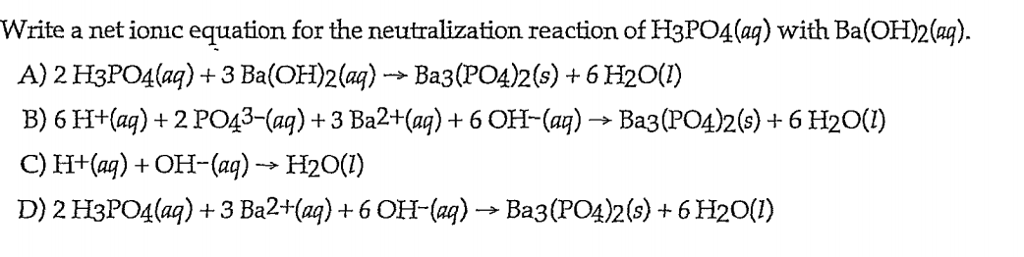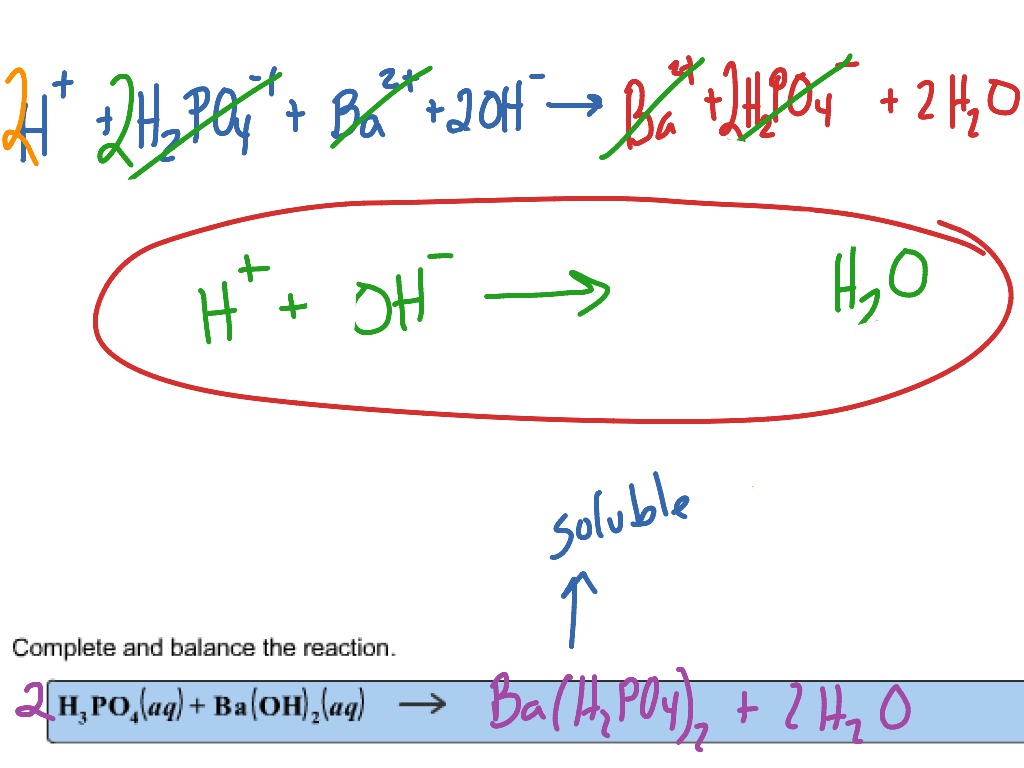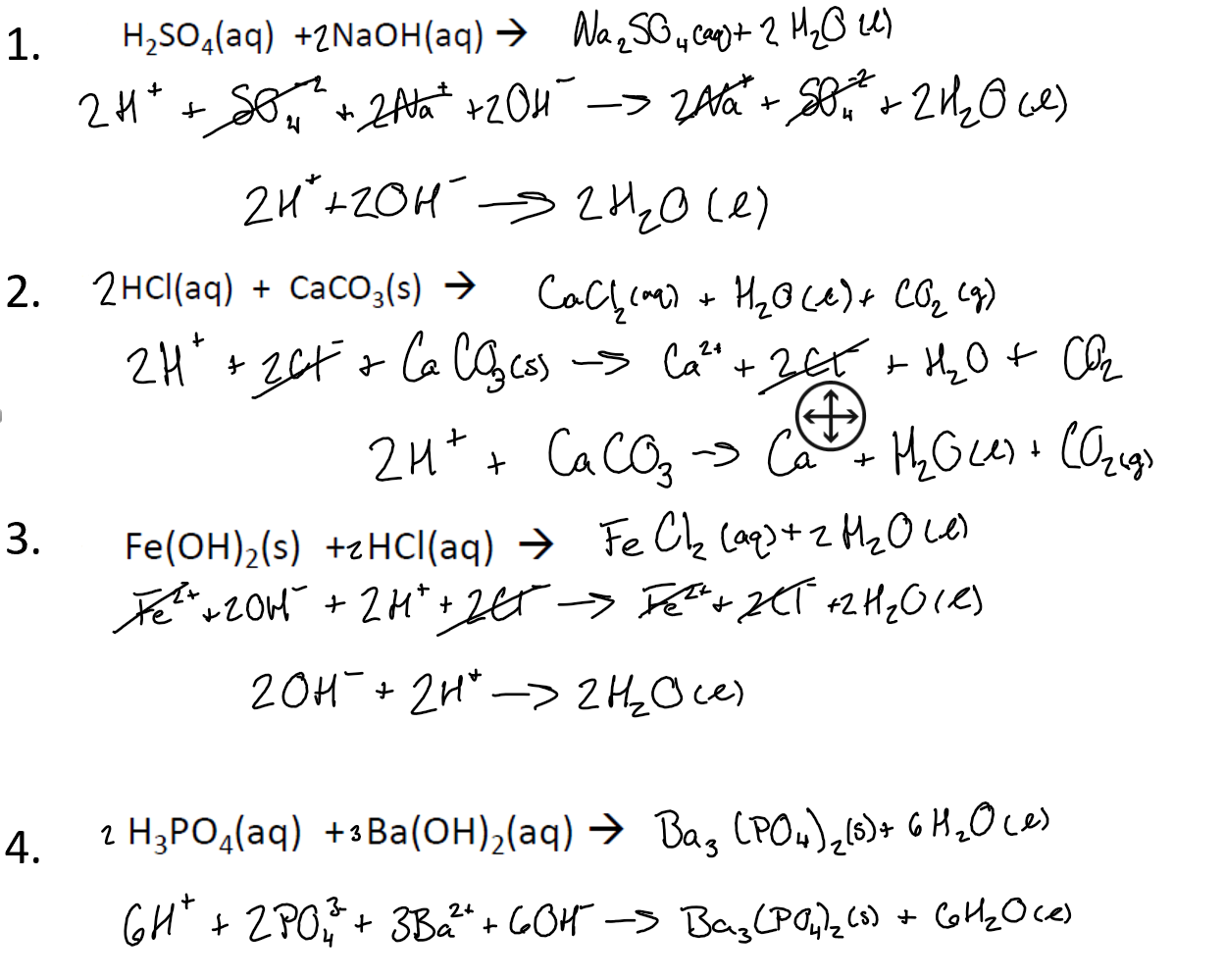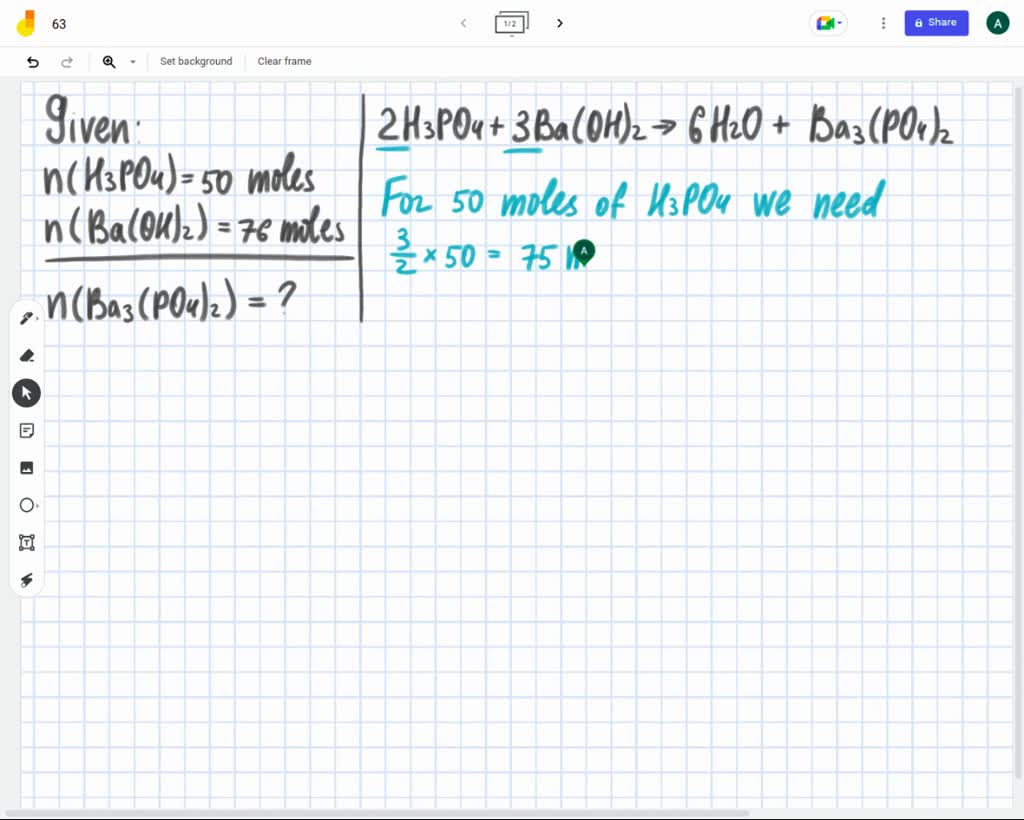
SOLVED: Given the balanced reaction, 2 H3PO4(aq)+ 3 Ba(OH)2(aq)> 6 H2O(l)+ Ba3(PO4)2(s), how many moles of Ba3(PO4)2(s) will be produced from a reaction mixture composed of 50 moles of H3PO4and 76 moles
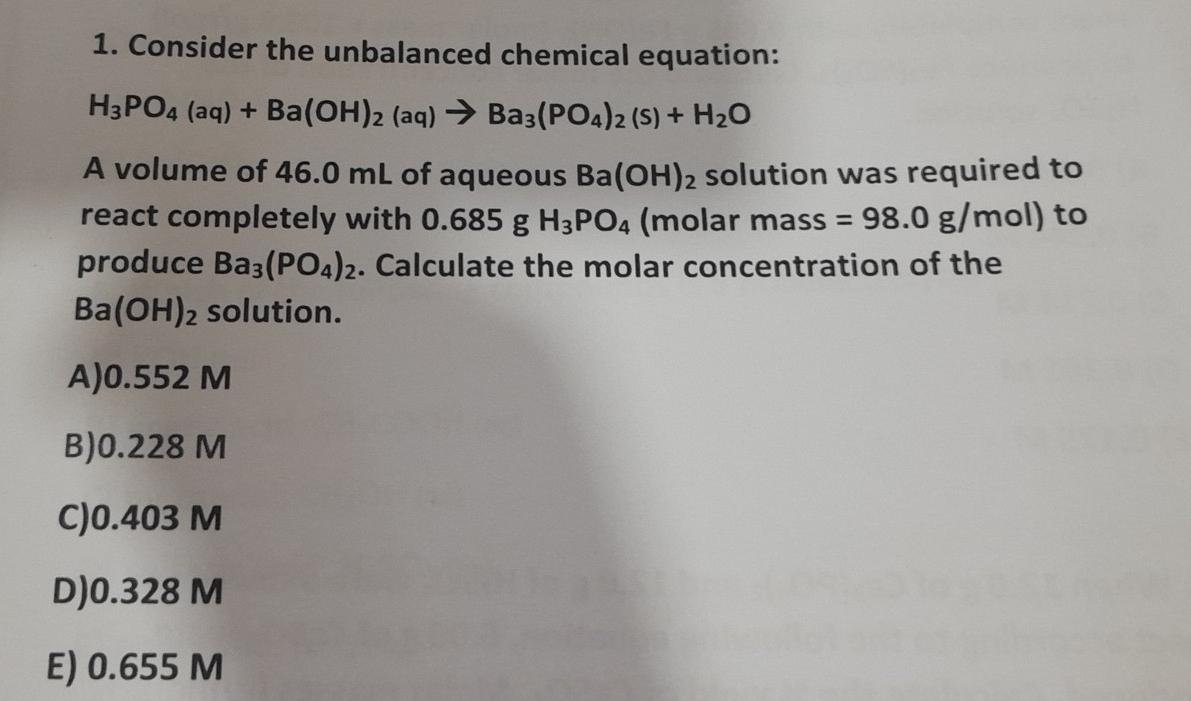
✓ Solved: What volume of 0.0521 M Ba(OH) 2 is required to neutralize exactly 14.20 mL of 0.141 M H 3...
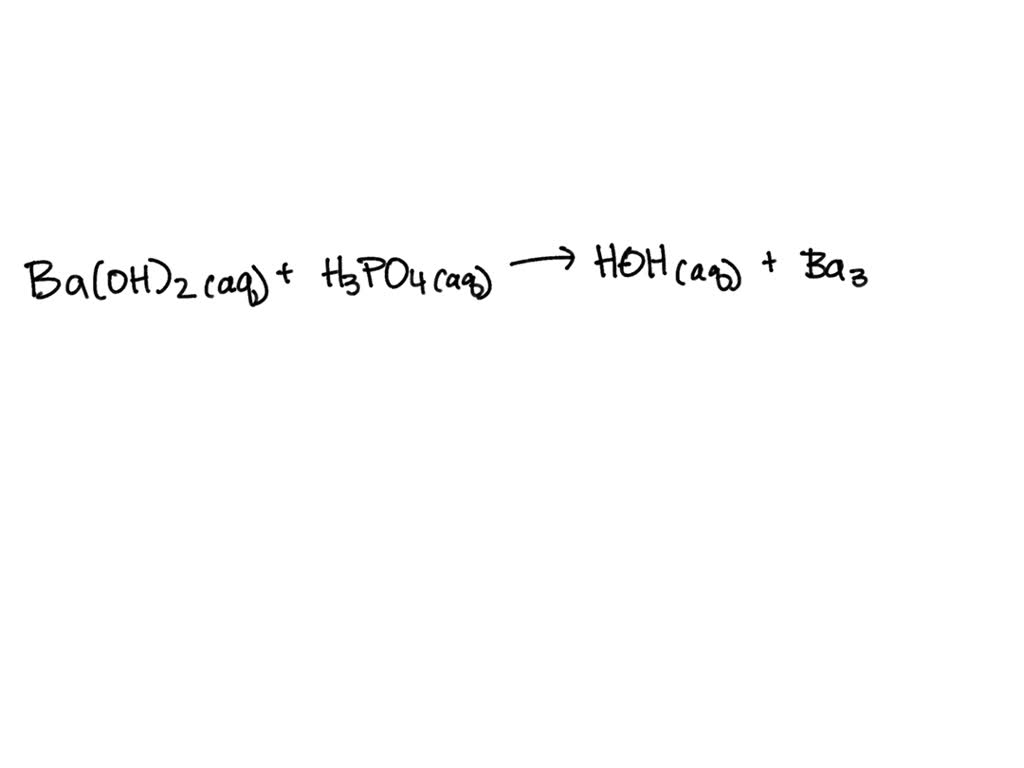
SOLVED: When a solution containing barium hydroxide is added to a solution containing phosphoric acid (HaPO4), a white precipitate forms and settles to the bottom of the beaker: What is the white