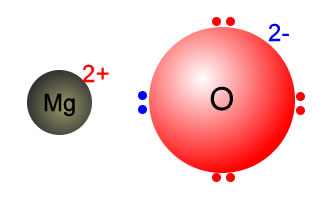
Why MgO exist as Mg^(2+)O^(2-) not as Mg^(o+)O^(ɵ) whereas the formation of Mg^(2+) from Mg requires more energy than formation of Mg^(o+) and formation of O^(ɵ) from O is exothermic whereas the
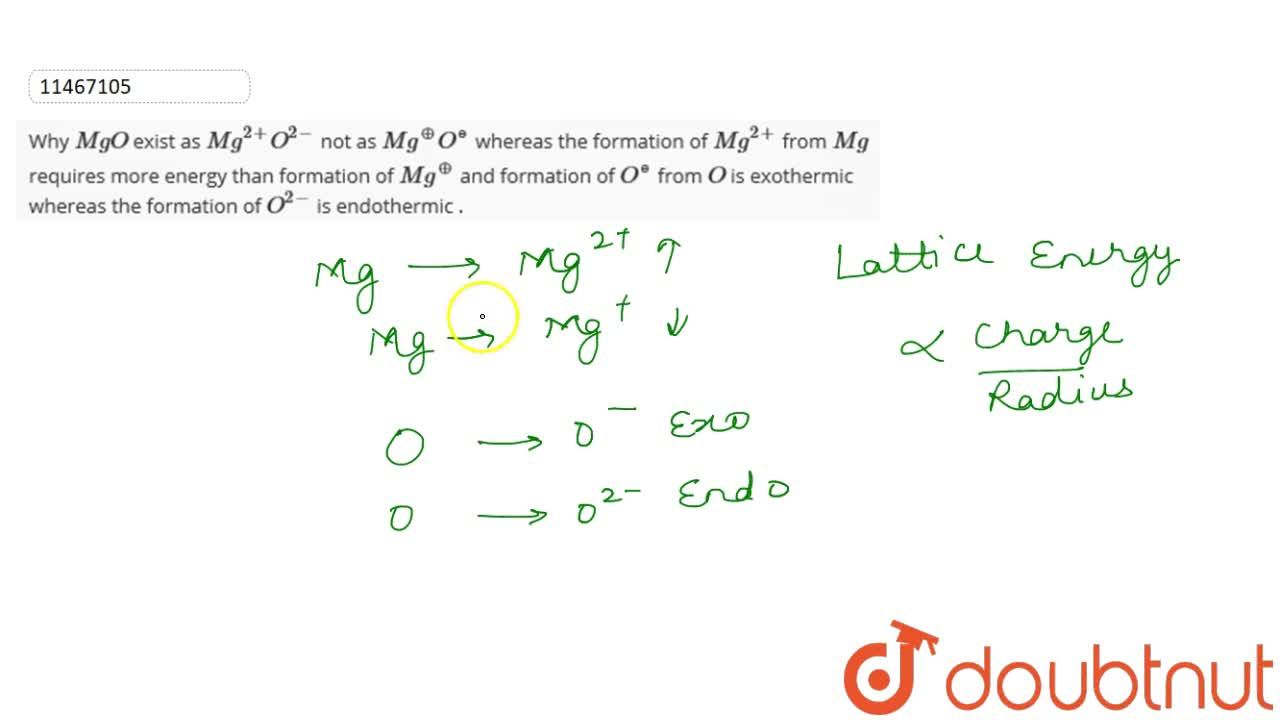
Why MgO exist as Mg^(2+)O^(2-) not as Mg^(o+)O^(ɵ) whereas the formation of Mg^(2+) from Mg requires more energy than formation of Mg^(o+) and formation of O^(ɵ) from O is exothermic whereas the

SOLVED:Consider the following energy changes: (TABLE CANNOT COPY) a. Magnesium oxide exists as Mg^2+ O^2-, not as Mg^+ O^- . Explain. b. What experiment could be done to confirm that magnesium oxide

New Insights of the Fenton Reaction Using Glycerol as the Experimental Model. Effect of O2, Inhibition by Mg2+, and Oxidation State of Fe | The Journal of Physical Chemistry A
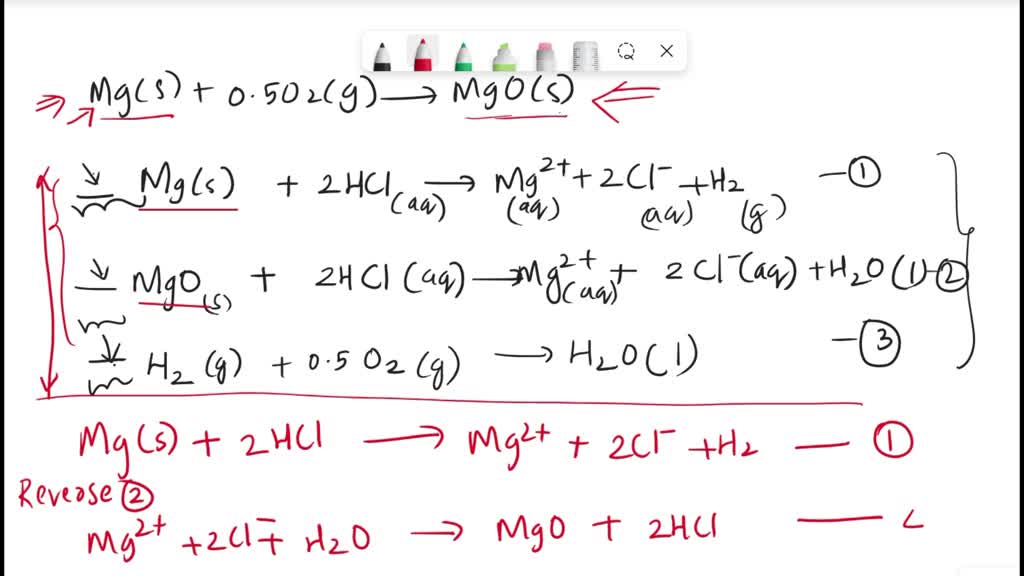
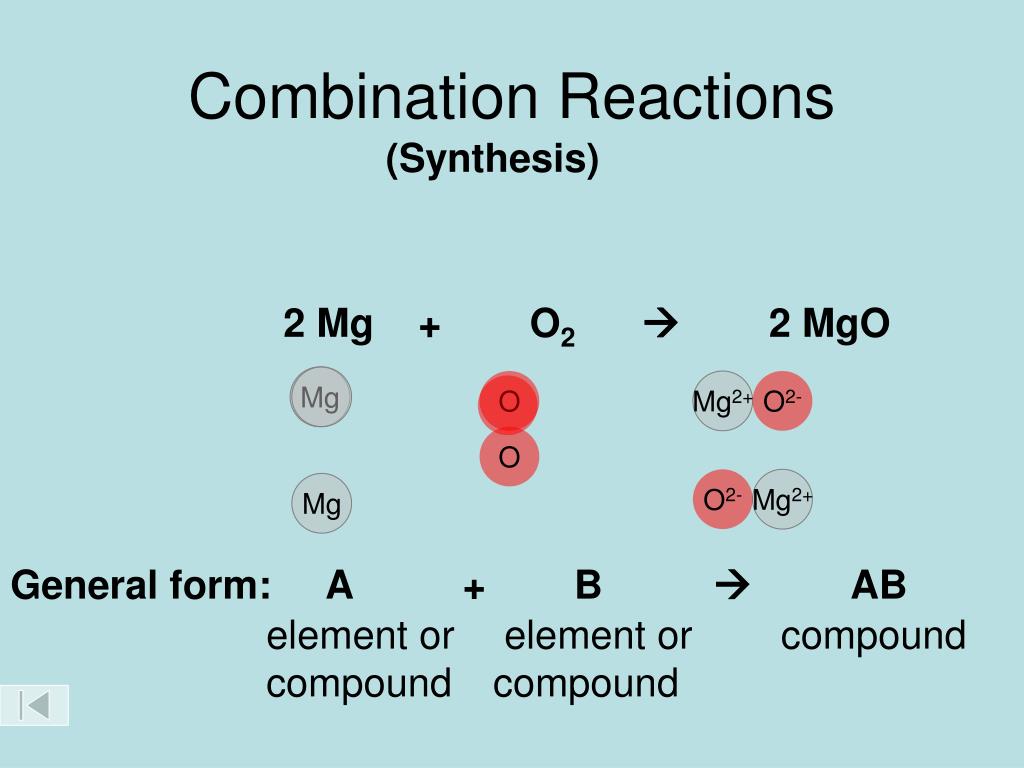
SOLVED: The formation reaction of magnesium oxide is as follows. Mg(s) + 0.5 O2(g) → MgO(s) This reaction can be constructed from the following reactions. In the blanks before each reaction, add