Explain in why on addition of 1 mole of NaCl to 1L of water, the boiling point of water increases, while addition of 1 mole of methyl alcohol to 1 L of

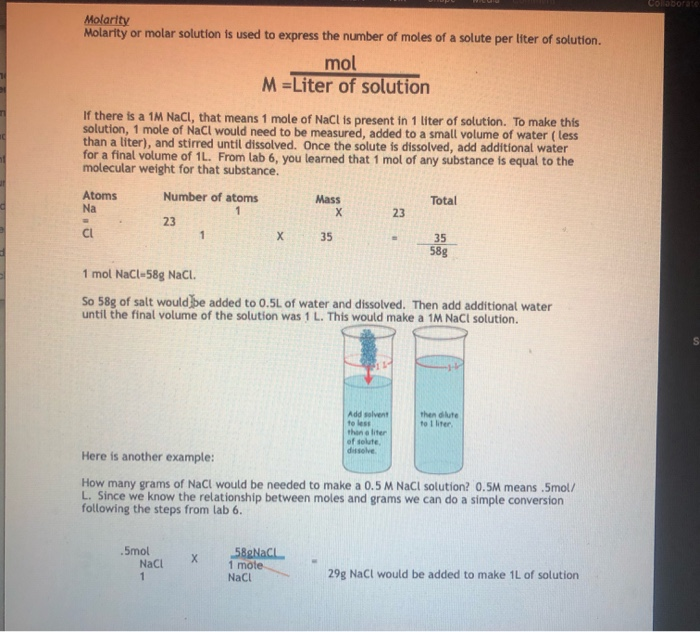
Concentration Calculations Molarity. Objectives To calculate the molecular weight and moles of a substance To calculate the Molarity of a substance using. - ppt download

SOLVED: Adding 1 mol of NaCl to 1 kg of water lowers the vapor pressure of water more than adding 1 mol of C6H1zOb. Explain: 01) NaCl is highly polar whereas CoH1zOs

Effects of 1 mol/L NaCl and the 1 mol/L NaNO3 electrolytes on surface... | Download Scientific Diagram

The Mole & Chemical Quantities. The Mole Mole-the number of particles equal to the number of atoms in exactly 12.0 grams of carbon mol = 6.02 x. - ppt download
If relative decrease in vapour pressure is 0.4 for a solution containg 1 mol of nacl in 3 mol of water then

Changes of phenol concentration with time for I NaCl = 1 mol kg −1 (a)... | Download Scientific Diagram
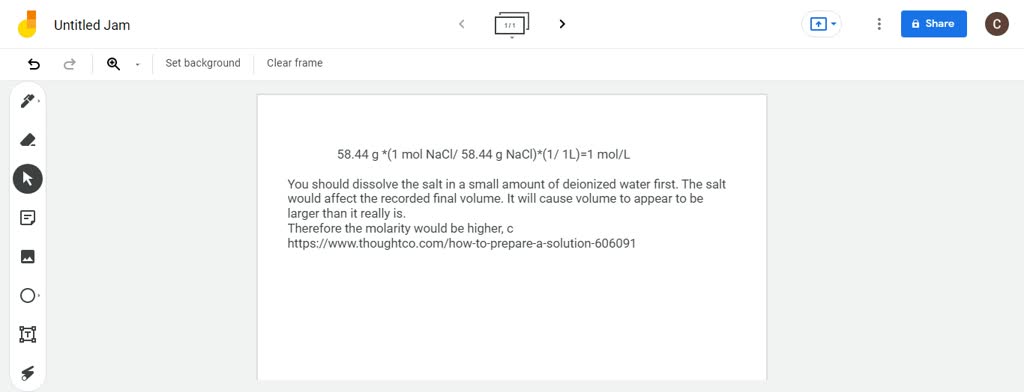
SOLVED:You prepared a NaCl solution by adding 58.44 g of NaCl to a 1 -L volumetric flask and then adding water to dissolve it. When finished, the final volume in your flask

Formula mass of NaCl is 58.45 g mol ^-1 and density of its pure form is 2.167 g cm ^-3 . The average distance between adjacent sodium and chloride ions in the

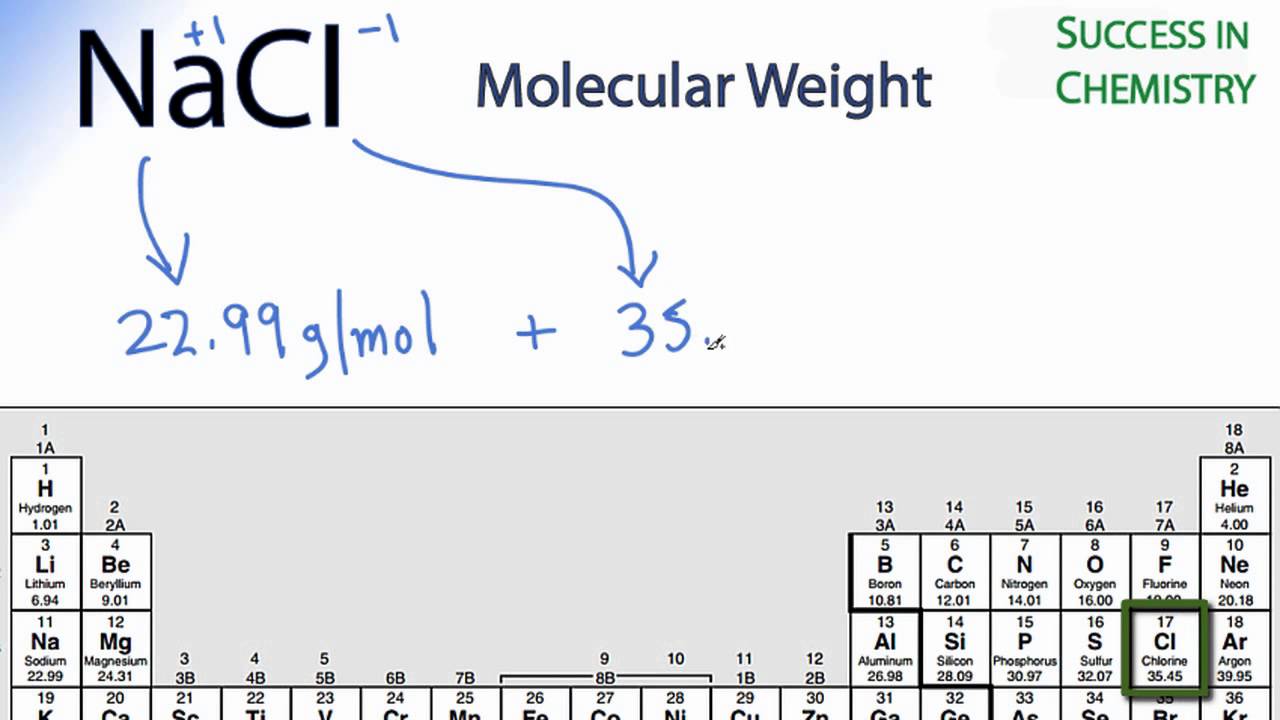
3/4/2016 I ObjectiveDo Now Convert grams of a substance to moles of a substance. Calculate the molar mass of: NaCl MgCl ppt download